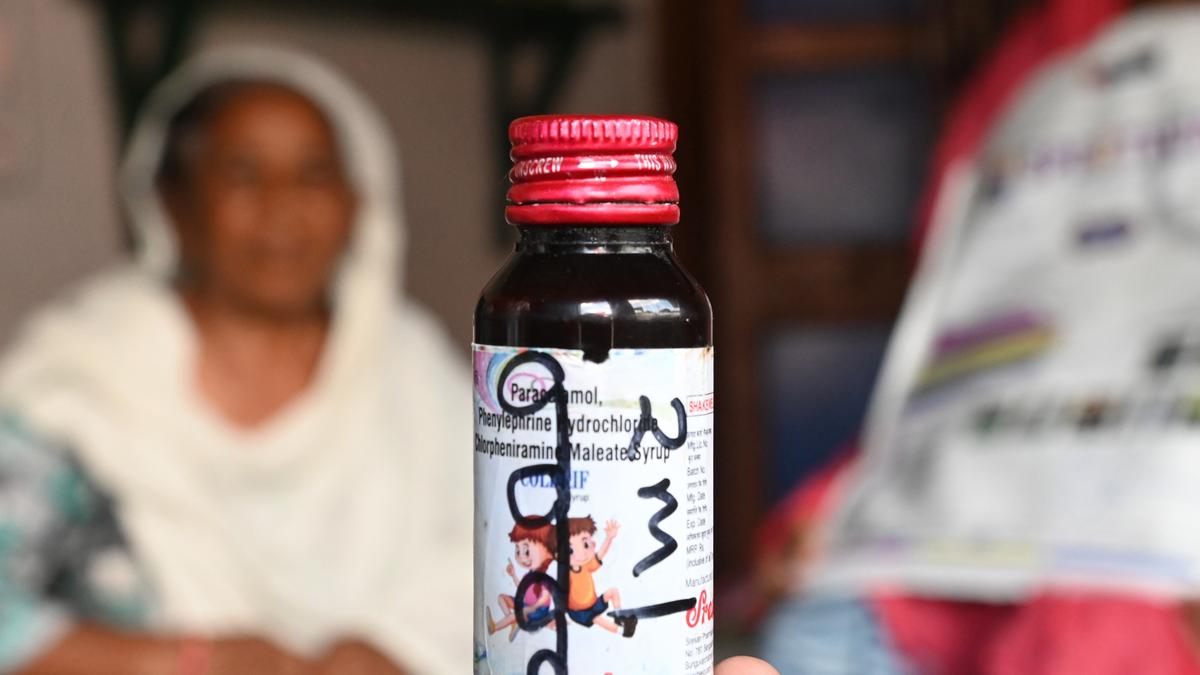
A bottle of the ‘Coldrif’ cough syrup, manufactured by Tamil Nadu-based Sresan Pharmaceuticals | Photo Credit: The Hindu
The State Drug Control Department officers had throughout an inspection discovered the cough syrup contained 48.6% of Diethylene Glycol (DEG), a poisonous substance. This medication is linked to the dying of kids in Madhya Pradesh.
GROUND ZERO | Killer cough syrup
The officers had additionally discovered that the corporate lacked correct good manufacturing practices (GMP) and good laboratory practices (GLP), and so they recorded over 300 vital and main violations.
The firm’s proprietor, G. Ranganathan, was not too long ago arrested by a particular investigation group from Madhya Pradesh.
Also Read | WHO seeks clarification from India if cough syrup has been exported to different nations
Earlier within the day, a group from the Enforcement Directorate searched the premises of Sresan Pharmaceuticals and a few officers, in a Prevention of Money Laundering Act (PMLA) case.
“The drug manufacturing license of Sresan Pharmaceuticals has been utterly cancelled, and the corporate has been closed. Orders have been given to conduct an in depth inspection of different drug manufacturing corporations situated in Tamil Nadu,” the federal government mentioned.
Published – October 13, 2025 01:40 pm IST



Leave a Comment